COMPUTATIONAL THERMOCHEMISTRY OF METAL COMPLEXES WITH N-BENZOYL-N',N'-DIALKYLUREA DERIVATIVES
J. R. B. Gomes, M. A. V. Ribeiro da Silva
Centro de Investigação em Química, Departamento de Química da Faculdade de Ciências da Universidade do Porto, Rua do Campo Alegre, 687, 4169-007 Porto (Portugal)
Acylcalcogenourea ligands are known to be useful ligands for the potential determination of transition metal (TM) traces by means of normal phase chromatography[1] and have been shown to selectively extract TM in the form of stable neutral metal chelates[2]. However, structural and energetic information about these metal complexes is scarce and conclusions are not straightforward[3]. To fulfil lack of knowledge about geometry and energetics of metal-ligand bonding, density functional (DF) calculations were carried out on complexes schematically shown in Figure 1.
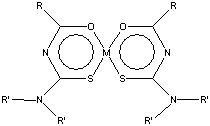
Figure 1: Structural model for [dialkylacylthioureatoM(II)] complex; M=Ni, Cu, Zn; R= -C6H5, -CnH2n+1; R'= -CnH2n+1.
In this communication, we will present geometric and energetic results computed using different basis sets and DF approaches that will be compared with available experimental data[3].
Thanks are due to the Funda��o para a Ci�ncia e Tecnologia (FCT) for financial support to Centro de Investiga��o em Qu�mica. JRBG thanks FCT for the award of a scholarship ref. BPD/22098/99
[1]- M. Schuster, Fresenius Z. Anal. Chem. 1992, 342, 791.
[2]- K. K�nig, M. Schuster, G. Schneeweiss, B. Steinbrech, Fresenius Z. Anal. Chem. 1985, 325, 621.
[3]- E. Guillon, I. D�champs-Olivier, J. -P. Barbier, Polyhedron 1998, 17, 3255; V. R. Richter, L. Breyer, J. Kaiser, Z. anorg. all. Chem. 1980, 461, 67; M. A. V. R. Silva, M. D. M. C. R. Silva, L. C. M. Silva, F. Dietze, E. Hoyer, Thermoch. Acta 2001, 378, 45.